Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -dispersed LATP by means of neutron radiography
-dispersed LATP by means of neutron radiographySong, F.*; Chen, H.*; Hayashida, Hirotoshi*; Kai, Tetsuya; Shinohara, Takenao; Yabutsuka, Takeshi*; Yao, Takeshi*; Takai, Shigeomi*
Solid State Ionics, 377, p.115873_1 - 115873_6, 2022/04
Times Cited Count:2 Percentile:28.85(Chemistry, Physical)Sakuma, Hiroshi*; Tachi, Yukio; Yotsuji, Kenji; Suehara, Shigeru*; Arima, Tatsumi*; Fujii, Naoki*; Kawamura, Katsuyuki*; Honda, Akira
Clays and Clay Minerals, 65(4), p.252 - 272, 2017/08
Times Cited Count:2 Percentile:16.47(Chemistry, Physical)Structure and stability of montmorillonite edge faces (110), (010), (100), and (130) of the layer charges y = 0.5 and 0.33 are investigated by the first-principles electronic calculations based on the density functional theory. Stacking and single layer models are tested for understanding the effect of stacking on the stability of montmorillonite edge faces. Most stacking layers stabilize the edge faces by making hydrogen bonds between the layers; therefore, the surface energy of stacking layers is reduced rather than the single layer model. This indicates that the surface energy of edge faces should be estimated depending on the swelling conditions. Lowest surface energies of (010) and (130) edge faces were realized by the presence of Mg ions on the edge faces. These edge faces have a strong adsorption site for cations due to local negative charge of the edges.
Arima, Tatsumi*; Idemitsu, Kazuya*; Inagaki, Yaohiro*; Kawamura, Katsuyuki*; Tachi, Yukio; Yotsuji, Kenji
Progress in Nuclear Energy, 92, p.286 - 297, 2016/09
Times Cited Count:12 Percentile:71.23(Nuclear Science & Technology)Diffusion and adsorption behavior of uranyl (UO ) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO
) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO , K
, K , CO
, CO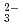 and Cl
and Cl and H
and H O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO
O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO is the smallest and is 26% less than the self-diffusion coefficient of H
is the smallest and is 26% less than the self-diffusion coefficient of H O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO
O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO CO
CO and UO
and UO (CO
(CO )
) can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO
can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO and K
and K were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO
were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO might be smaller than that of K
might be smaller than that of K . Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
. Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
Yamada, Hirohisa*; Yokoyama, Shingo*; Watanabe, Yujiro*; Morimoto, Kazuya*; Suzuki, Shinichi; Yaita, Tsuyoshi; Hatta, Tamao*
no journal, ,